The electronegativity difference between nonmetals is relatively small so such compounds are nearly always covalent. For this purposes a dimensionless quantity the Pauling scale symbol χ is the most commonly used.
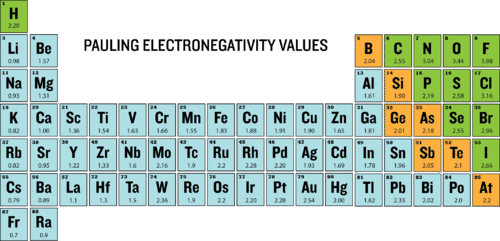
Periodic Trends Electronegativity Ck 12 Foundation
The electronegativities of nonmetals are generally high.
. The value of electronegativity is assigned using a relative scale on which _____ the most electronegative element has an electronegativity of 40. The larger the difference in electronegativity between two atoms the more polar the bond between them. Broadly they lack a preponderance of more metallic attributes such as luster deformability good thermal and electrical conductivity and low.
Bonding between a metal and a nonmetal is often ionic. Some compounds contain both covalent and ionic bonds. Such as between two different nonmetals then the bond is _____ nonmetals.
Since metals have few valence electrons they tend to increase their stability by losing electrons to become cations. Of the nonmetals fluorine is the most electronegative followed by oxygen nitrogen and chlorine. The electronegativity of nonmetals is relatively _____ as compared to the electronegativity of metals.
Solniwko 45 1 year ago. Question 6 5 pts Al P Na He C N Which of the following elements would be expected to have the highest electronegativity. Therefore metals are electropositive and non-metals are electronegative in nature.
Electronegativity - relative ability of an atom to attract electrons to itsef in a chemical compound - elements with high electronegativites tend to acquire electrons in chemical reactions and are found in the upper right corner of the periodic table. Nonmetals have much higher electronegativities than metals. - elements with low electronegativities tend to lose electrons in chemical reactions and are found in the lower.
The larger the difference in electronegativity between two atoms the more polar the bond between them. The electronegativity of nonmetals is relatively _____ as compared to the electronegativity of metals. Nonmetals have much higher electronegativities than metals.
The electronegativity of nonmetals is relatively _____ as compared to the electronegativity of metals. Question 7 5 pts decreases increases Generally speaking in the periodic table electronegativity decreases increases when. Nonmetals have more valence electrons and increase their stability by gaining electrons to become anions.
Nonmetals have much higher electronegativities than metals. A nonmetal is a chemical element having among other properties a relatively low density and moderate to high electronegativity. Electronegativity of Iron is 183.
Bonds between two nonmetals are generally covalent. Higher Electronegativity generally increases from the left of the PTmetals to the right nonmetals. The larger the difference in electronegativity between two atoms the more polar the bond between them.
This bond is usually formed between two nonmetals since there is relative low difference in electronegativity. For more information on this visit. All nonmetals share some common chemical properties such as relatively high electronegativity allowing nonmetals to obtain electrons when they react with metals to form ionic compounds.
Nonpolar covalent bond when electrons of a bond are shared equally between atoms the bond is called. Consequently the electronegativities of metals are generally low. Of the nonmetals fluorine is the most electronegative followed by oxygen nitrogen and chlorine.
Following these rules the non-metals which are organized on the right side of the periodic table have higher electronegativity values than the metals. Question 7 5 pts increases increases decreases increases increases decreases decreases decreases. The element with the highest.
Of the nonmetals fluorine is the most electronegative followed by oxygen nitrogen and chlorine. Electronegativity symbol χ is a chemical property that describes the tendency of an atom to attract electrons towards this atom. The atoms in polyatomic ions such as OH NO 3 and NH 4 are held.
It is a general observation that metals show a lower value of electronegativity as compared to the non-metals. As the electronegativity difference increases between two atoms the bond becomes more ionic. While Non metals tends to have negatively charged ions as like.
We have that The electro negativity of nonmetals is relatively High as compared to the electro negativity of metals which is Low. Question 6 5 pts Na Al N He C P Which of the following elements would be expected to have the highest electronegativity. On the other hand the electronegativity difference between a metal and a.
The electronegativity of Iron is. The elements in period two differ in properties from their respective group elements due to the small size and higher value of electronegativity. What is electronegativity.

Electronegativity Table Easy Hard Science

0 Comments